Key Points:
- Nitrogen is a plant-essential macro-nutrient that supports critical plant functions, plant development, and grain yield.
- Understanding the nitrogen cycle is fundamental to managing nitrogen as a system.
- The nitrogen cycle is dynamic and involves several mechanisms for nitrogen transformation.
- The primary mechanisms for nitrogen loss from the cropping system are leaching, denitrification, volatilization, and runoff/erosion.
The Role of Nitrogen in Plant Metabolism
Nitrogen is an essential macro-nutrient required in large quantities for optimum plant growth and development, comprising about 1-5% of total plant dry matter. Nitrogen is a key building block of amino acids, which form structural plant proteins and dozens of different enzymes that regulate plant function. Nitrogen is an inherent component in the chemical structure of chlorophyll, which is required to drive photosynthesis and manufacture carbohydrates. Optimum grain yield clearly depends on having an adequate supply of nitrogen at critical periods in the growth and development of the plant.
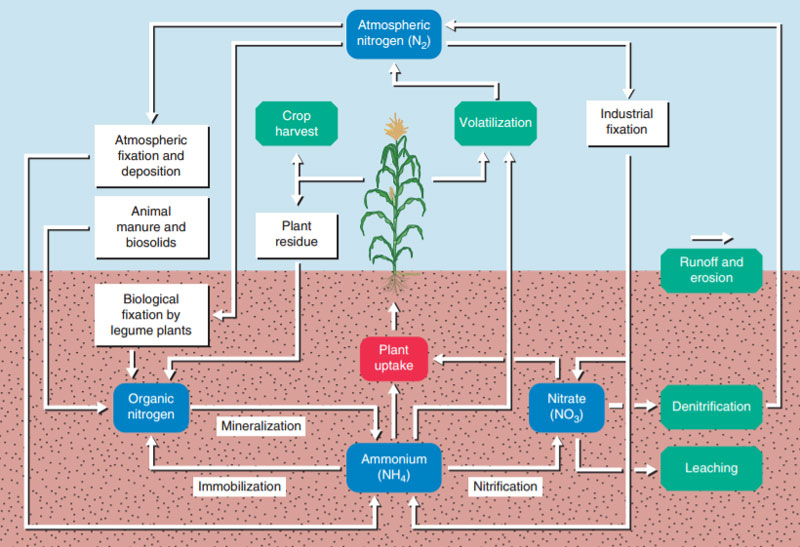
Key Processes in the Nitrogen Cycle
Understanding how to manage nitrogen in the cropping system depends on a strong fundamental understanding of the key processes involved in the nitrogen cycle.
- Biological Fixation. Nitrogen gas (N2) comprises 78% of the atmosphere of earth and represents a major source of nitrogen to support plant life. Symbiotic bacteria work to naturally fix atmospheric nitrogen and make it available to legume plants.
- Industrial Fixation. Nitrogen fertilizers are manufactured by using fossil fuel energy to capture atmospheric nitrogen into forms that can be easily applied to the cropping system. These additional nitrogen inputs supplement natural nitrogen cycling and replace nitrogen removed as grain.
- Immobilization. Nitrogen in organic plant materials or manure is immobilized by soil microbes into a stable source of soil nitrogen called organic matter. This nitrogen is temporarily unavailable to the plant, but is also much less subject to loss from the system. Fertilizer nitrogen may also be immobilized into microbial biomass in the presence of high carbon residues, as the bacteria incorporate nitrogen into their cells while consuming the organic residue.
- Mineralization (Ammonification). Nitrogen in organic matter is mineralized and released by soil microbes to make it available to growing plants. The amount of natural nitrogen mineralization varies according to soil organic matter, soil pH, rainfall, temperature, and other seasonal variables, making prediction of actual mineralized nitrogen somewhat challenging. Mineralization contributions to available nitrogen are typically estimated based on organic matter content of the soil and fertilizer recommendations adjusted accordingly.
- Nitrification. Soil bacteria Nitrosomonas and Nitrobacter mediate the biological transformation of ammonium (NH4+) to nitrite (NO2-) and then nitrate (NO3-), respectively. These transformations occur more rapidly as soil temperatures exceed 50 F. Ammonium ions are readily adsorbed by negatively charged soil surfaces and are much less subject to loss when incorporated into the soil. Nitrate ions are soluble and repelled by the positively charge soil surfaces, making nitrate more prone to loss. Nitrification inhibitors, like nitrapyrin and DCD, can be used with ammonium fertilizers to slow down the nitrification process, improving retention of nitrogen in the soil system and preventing loss.
- Plant Uptake (Assimilation). Ideally, a high percentage of applied nitrogen is taken up by the plant and assimilated into plant biomass. Both ammonium and nitrate ions are able to be absorbed by the plant. Nitrate is usually the dominant form in the soil, but is actually the less efficient form for plant uptake, requiring 50% more plant energy to reduce the chemical species back to the ammonium form in the roots or shoots for further assimilation into proteins. Each year, significant quantities of nitrogen are removed from the system as harvested grain.
Primary Loss Mechanisms of Fertilizer Nitrogen
- Leaching. Negatively charged nitrate ions can be leached from the soil profile by the natural downward movement of water. In exceedingly wet years, leaching losses can be significant, particularly in well-drained soils or artificially drained tile systems. Leached nitrates can end up in drinking water supplies and must be removed at great expense to ensure water quality standards.
- Denitrification. Most soil microbes are aerobic and require oxygen to respire, just like people. Under saturated soil conditions and warm temperatures, certain bacteria called facultative anaerobes can alternately use the oxygen in nitrate to respire, converting the nitrate to nitrous oxide gas (N2O) that can escape the soil system to the atmosphere. In typical medium-to-heavy textured Midwest soils, denitrification is the primary mechanism of nitrogen loss.
- Volatilization. Fertilizers containing ammonium or urea are also subject to nitrogen loss via volatilization of free ammonia (NH3) gas from the soil surface to the atmosphere. Losses are greater with moist soil, high soil pH, high soil temperatures, and high surface residue. As an example, volatilization losses from urea can exceed 50% without proper management, like incorporation or use of urease inhibitors.
- Runoff and Erosion. Ammonium ions adsorbed to soil particles or other surface-applied fertilizers can be subject to physical loss by soil erosion or surface runoff. These losses are best prevented through soil conservation practices and incorporation of fertilizer materials.
4R Best Management Practices for Nitrogen
In the next article, we will begin to address the best management practices to minimize nitrogen loss, optimize uptake, drive maximum yield, and reduce our environmental impact. Key points will be nitrogen product form, placement, timing, and rates.
(Photo: University of Illinois)
Contact your FS Crop Specialist for your agronomic needs.